Experiment
1A: Measurement and Error
In most of our labs, you
measure something then compare to what is predicted by theory. These numbers are seldom equal, because
measurement is never exact. Today, we
look at the rules for deciding how close is close enough; whether the
difference is just what you should have expected.
Measurements can be
affected by several kinds of errors. One
kind is mistakes. Another is systematic
errors, due to some kind of bias in the measurements. Then there are errors due to experimental
uncertainty, the fact that no method of measuring is accurate to an infinite
number of decimal places. These errors tend
to be random, so they can be analyzed statistically, but unlike other kinds of
errors, they cannot be eliminated by being careful. Therefore, measurement does not give you a
precise value, but simply a range of possibilities. Notation:
4.73+.01 cm means that you've reasonably sure the true value lies
between 4.72 cm and 4.74 cm. (You don't
know where between 4.72 and 4.74 the exact number lies; 4.73 is just the middle
of that range. A best guess.)
If you do a calculation
using uncertain numbers, the result is also uncertain. (These rules can be derived by using
differentials to represent the uncertainties.
For details, ask the instructor.)
|
-
If adding or subtracting the measurements, add the uncertainties. (In the same units as
the measurements, not as percents.) -
If multiplying or dividing, convert the uncertainties to percents,
then add to get the answer’s percent uncertainty. -
With other operations, trig functions for example, find the differential of
the formula then throw away all minus signs. (If just starting Calculus, you will learn
about differentials soon.) |
HOW TO DO PERCENTS: I am always
amazed how, in a room full of people taking calculus, no one knows how to do percents. Remember
this is here so you can refer back to it during future labs.
To find what one number is as a percentage of another, divide
(and multiply by 100):
Example: 3.5 +
.2 cm (.2 / 3.5) x 100% = .057
x 100% =
5.7%
3.5 cm + 5.7%
To find some percentage of a number, multiply:
Example:
3.5 cm + 5.7% 5.7%
of 3.5 cm = (.057)(3.5 cm) = .2 cm
3.5 + .2 cm
Part 1: Volume of a Solid Cylinder.
You will check whether the
formula V= πr2h gives the correct volume for a solid
plastic cylinder.
1. With a ruler, measure its diameter and height, estimating
an uncertainty based on how closely you can read the ruler. Calculate the volume from the formula.
2. Determine the volume’s uncertainty with the rules
given above. Imitating this example will
help.
Example: Find the volume
of a cylinder of diameter 1.80 + .05 cm and height 2.50 + .05 cm.
![]()
![]() x 100% = 2.8%
x 100% = 2.8%
![]() Answer: r = ˝ d = (.5 +
0%)(1.80 + 2.8%) = .90 + (0 + 2.8)% = .90 cm + 2.8%
Answer: r = ˝ d = (.5 +
0%)(1.80 + 2.8%) = .90 + (0 + 2.8)% = .90 cm + 2.8%
The ˝ is not a measured number, so it is not uncertain.
Notice the radius has the
same percent uncertainty as the diameter, not the same number of cm.
V= πr2h = (3.14 … +
0%)(.90 + 2.8%)(.90 + 2.8%)(2.50 + 2.0%)
= 6.3617 … + (0 + 2.8 + 2.8 + 2.0)%
![]() = 6.3617 … + 7.6%
= 6.3617 … + 7.6%
7.6%
of 6.3617 = (.076)(6.3617) = .4835 Rounding, V = 6.4 + .5 cm3
Notice that because r is squared, its
percent uncertainty is counted twice.
The answer is rounded to
the nearest tenth because the last
significant figure is the one which is somewhat, but not completely, uncertain. With an uncertainty of a few tenths, we have
some vague information at that level. We
have no idea what goes in the hundredths place.
3. Put some water
in a 100 ml graduated cylinder and record its volume, estimating an
uncertainty. Add the solid cylinder and
record the total volume of water plus cylinder.
Subtract to get the volume of the solid cylinder, using the rule from
the previous page to get the uncertainty.
4. In your
conclusion, state whether the volume measured by displacing water agrees with
the volume calculated by the formula.
That is, do the two ranges of possible values have some common
ground? For example, 3.0 + .2
agrees with 3.21+.02, but 3.0 + .1 does not agree with 3.21+.02.
Part 2: Volume of
a metal block.
You will repeatedly measure the volume of a metal block,
using a more accurate instrument each time.
The values you get for the volume should all agree with each other,
within their uncertainties.
From the set of five blocks, select one. You will not use the others. (Do not put it in water.)
a. Ruler. Measure
the block’s length, width and height with a ruler. If the 0 mark isn't clear, you may find it's
better to put the edge of the block at the 1 cm mark, then subtract 1 cm from
the reading. Read the ruler as
accurately as possible, estimating tenths of a millimeter. Estimate a reasonable uncertainty for each
measurement. Calculate a best value for
V. Use the rules in the box to find V's
uncertainty. Remember to include units
on all values.
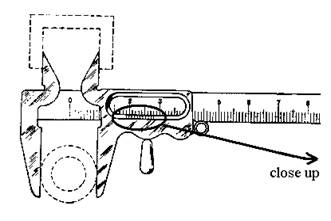
b. Vernier Caliper.
(See diagram, next page.) To read
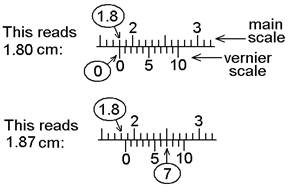
a vernier caliper:
a. Read how many whole
millimeters you have from the main scale.
b. Read tenths of a
millimeter from the vernier scale by looking for
which mark best lines up with one on the main scale. A magnifying glass is available, if it would
help you see the marks.
 Measure the block’s dimensions, reading the instrument
as accurately as possible, and estimate the uncertainty. Calculate V and its uncertainty.
Measure the block’s dimensions, reading the instrument
as accurately as possible, and estimate the uncertainty. Calculate V and its uncertainty.
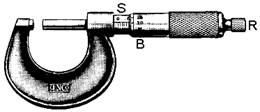 c. Micrometer.
c. Micrometer.
The screw inside has a half-millimeter thread, so one
turn advances the jaw and also the barrel B, .5 mm. The barrel has 50 marks around it, so turning
it by one division moves the jaw .01 mm.
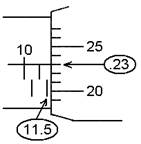 To take a measurement, close the jaws onto the object
until there just starts to be a little resistance. DO NOT OVERTIGHTEN. Add the reading from the main scale to the
reading from the barrel. In the example
shown, 11.5 mm from the main scale would be added to .23 mm from the barrel,
making the reading 11.73 mm (or 1.173 cm).
To take a measurement, close the jaws onto the object
until there just starts to be a little resistance. DO NOT OVERTIGHTEN. Add the reading from the main scale to the
reading from the barrel. In the example
shown, 11.5 mm from the main scale would be added to .23 mm from the barrel,
making the reading 11.73 mm (or 1.173 cm).
Check that your micrometer is correctly zeroed (they get
out of adjustment fairly easily) then repeat the measurements of width and
thickness.
The micrometer does not open wide enough to measure its
largest dimension; re-use your value from the vernier
caliper. Once again calculate V with its
uncertainty.
As your conclusion, answer the following:
-
Does V from the vernier caliper agree with V from the ruler?
-
Does V from the micrometer
agree with V from the ruler?
-
Does V from the micrometer
agree with V from the vernier caliper ?
If your V's do not agree, you've made a mistake. Find and correct it. One possibility is that your estimated
uncertainties might not have been very good estimates.
Notice that the increasing accuracy of the instruments
only reduces, does not eliminate, experimental errors. Any number based on measurement has at least
a small range of uncertainty.
PHY
131 Experiment
1A: Measurement & Error
Reminders:
1. Hand labs in before leaving unless I sign
them.
2. Work in pencil (or work out mistakes on a
first draft.)
3. Write a discussion of
the experiment (Objective/Procedure/Conclusion) to hand in with this answer
sheet. Don't forget to put your name on
it.
Part 1:
diameter = __________ +
__________ height = __________ +
___________
Show calculation of V and its cm3 of uncertainty:
Grad. cylinder: V of water = _________ + ________ V water + cyl. =
__________ + _________
V of cylinder & its
uncertainty:
Part 2:
a. Ruler: Dimensions: _________
+ _______, _________ +
_______, _________ + _______
Calculate V and uncertainty:
b. Vernier Caliper:
_________ + _______, _________ +
_______, _________ + _______
Calculate V and uncertainty:
Use the back for (c)
Micrometer.