Experiment 13: Spectroscopy
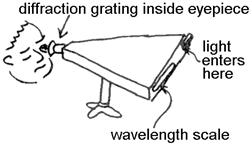 APPARATUS: Light enters the spectroscope through a small
opening, falls on a diffraction grating inside, and finally goes to your
eye. As the light passes through the
grating, different colors are bent into different directions, making them
appear at different points on the wavelength scale in the background. The scale is calibrated in nanometers.
APPARATUS: Light enters the spectroscope through a small
opening, falls on a diffraction grating inside, and finally goes to your
eye. As the light passes through the
grating, different colors are bent into different directions, making them
appear at different points on the wavelength scale in the background. The scale is calibrated in nanometers.
Part
1: Continuous Spectrum.
Set
the long, clear light bulb on books or wooden blocks so that it lines up with
the opening into the spectroscope. Check
that the opening isn't all the way shut.
Place the opening very close to the bulb, and aim the instrument at the
bulb by slowly moving the spectrometer back and forth while looking into it.
Record
the range of wavelengths you can see.
Can some people see higher or lower wavelengths than others?
Part
2: Spectroscopy.
You
will see how an object's spectrum (how its light is distributed between
wavelengths) can be used to identify its composition.
You
have a stand holding a glass tube. When
you plug it in, an electric current goes through the gas in the tube, making it
glow.
CAUTION: The sockets that the ends of the glass tube
go in operate at 5000 V. If you handle
the tube, turn it off and also be sure it is not too hot to handle.
1. Put the spectroscope on books to get it the
same height as the narrow middle part of the glass tube. Then, aim it as you did in part one: Put the opening where light enters the
spectroscope about an inch from the tube, then while looking in the
spectroscope, slowly turn it one way or the other until you see bright colored
lines appear.
2.
Turn off the overhead lights. (You might
pick up spectral lines from them instead of from your own gas. The spectrum shows up better in a dark room,
too.) If the numbers are hard to read,
ask for a flashlight, and shine it on the white part of the spectroscope
labeled "wavelength scale" on the diagram above.
3. Look through the spectroscope, and record the
colors and wavelengths of all the bright lines you see.
4. Identify the gas by comparing what you saw to
the spectrum chart on the wall of the lab.
Hints:
a. Your gas is one of the two at the bottom of the chart,
or the two at the top: mercury, lithium, helium or hydrogen.
b. Go by the wavelengths printed on the chart rather than
how the colors look; some of the colors are not true to life. The numbers on the chart have an extra zero
on the end.
c. There might be nothing on the chart that's a perfect
match. You may have seen some faint
lines or a general background of color, caused by factors such as air having
leaked into the tube. Also, you could
have overlooked faint lines that were supposed to be there. But there should be a spectrum on the chart
containing all of the brighter lines you saw.
5. Have the instructor check whether you
identified the gas correctly, and assist you if you did not.
6. Ask the instructor which gas to use next –
one of the two needs to be hydrogen (for you to compare to in part 3.) Move to this setup and repeat the same
procedure.
Part
3: The Bohr model of hydrogen.
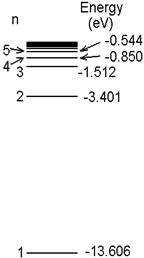
You
will compare the observed spectrum of hydrogen to that predicted by the Bohr
Model. The five lowest energy levels in hydrogen, according to the Bohr model,
are as shown in the diagram. The lines
in the visible part of the spectrum come from an electron dropping into the n =
2 energy level. Calculate the wavelength
of the first three of these lines as follows:
1. Find the energy lost by the electron as it
falls from 3 to 2.
2. Find the frequency of the photon it emits as
it does this, using E = hf.
3. Find the wavelength corresponding to the
frequency you found in step 2. (Use
300,000 nm/ps for the speed of light.)
4. Repeat steps 1 through 3 for an electron
falling from 4 to 2. Repeat them again
for 5 to 2.
Compare the hydrogen spectrum which you saw through the
spectroscope to what you calculated.
Report on experiment 13:
Spectroscopy
Name _____________________________________
Part 1:
Person #1: nm to nm, Person #2: nm to nm
Person #3: nm to nm, Person #4: nm to nm
Part 2I:
First
Gas: Second Gas:
Color Wavelength color Wavelength
______________
______________ ______________ ______________
______________
______________ ______________ ______________
______________
______________ ______________ ______________
______________
______________ ______________ ______________
______________
______________ ______________ ______________
______________
______________ ______________ ______________
______________
______________ ______________ ______________
Identify
gas: Identify
gas:
______________________ ______________________
Part 3:
3
to 2: 4 to 2: 5 to 2:
1.
2.
3.
Compare: