Experiment 6B: Electron
Diffraction
PURPOSE: To show that electrons are waves by measuring
their wavelength, thus verifying de Broglie's hypothesis. (Thomson's e/m experiment, which you did in
PHY 132, shows that electrons are particles by finding the charge to mass ratio
of each particle. Between the two
experiments, you will have demonstrated the dual wave-particle nature of the
electron.)
APPARATUS: Caution! Shock Hazard! Both the 50 V connections and 2500 - 5000 V
connections should be treated with respect.
There will be uninsulated connectors at several points.
You have an evacuated glass
bulb with an electron gun in one end.
Across the gun's exit is a thin layer of carbon (supported by a
micro-mesh grid of wires). As the beam
goes through this carbon, it is diffracted by two different sets of atomic
planes, forming two concentric circular fringes on the luminescent screen. Measuring this interference pattern gives an
experimental wavelength, which can be compared to de Broglie’s theory.
 Since the target is so thin, the beam can burn a hole in
it if too intense. You control the beam
intensity by adjusting the "bias voltage" applied across two parts of
the cathode. In addition to adjusting
this correctly, you should also inspect the target from time to time; if it
starts to glow dull red, shut down immediately. (The glow from the filament at
the other end of the gun is normal.)
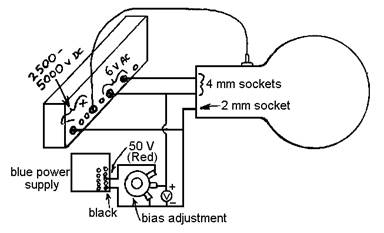
Make connections to the tube as shown.
Be sure that the negative connections are made to the smaller plug, as
shown. Have the instructor approve your
wiring before you turn anything on.
Since the target is so thin, the beam can burn a hole in
it if too intense. You control the beam
intensity by adjusting the "bias voltage" applied across two parts of
the cathode. In addition to adjusting
this correctly, you should also inspect the target from time to time; if it
starts to glow dull red, shut down immediately. (The glow from the filament at
the other end of the gun is normal.)
Make connections to the tube as shown.
Be sure that the negative connections are made to the smaller plug, as
shown. Have the instructor approve your
wiring before you turn anything on.
PROCEDURE:
With the high voltage all
the way down, turn on the 6 V AC. Wait
one minute for the cathode's temperature to stabilize. Then, set the bias voltage to 50 V.
Set the accelerating
voltage to about 4000 V. Slowly turn
down the bias voltage until rings appear around the central spot. To avoid damaging the target, keep this
pattern just bright enough to be seen.
(Turn out some of the overhead lights.)
Measure the diameters, D,
of both rings for several different voltages between 2500 V and 5000 V. (Measure to the center of the ring,
not its inner or outer edge.) You may
find using a compass as a pair of dividers more convenient than a ruler against
the curved surface. Estimate an
uncertainty in D. Readjust the bias each
time for minimum brightness. Avoid
touching the tube with your fingers while measuring; it distorts the ring
pattern by disturbing the static charge on the glass.
Shut off the high voltage
first, then the bias.
CALCULATIONS:
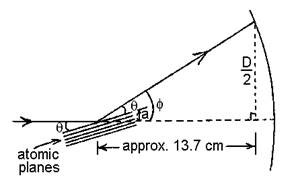
For each ring in each
trial, find f, the angle by which the
beam was deflected. (The picture is not
to scale. f is actually smaller.)

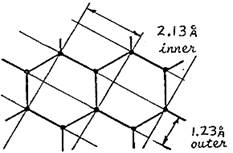 Both rings are due to first order Bragg
diffraction. The different diameters are
due to different distances between the planes doing the diffracting. The planes responsible for each ring are as
shown. (Graphite has a hexagonal atomic
arrangement.) Calculate the wavelength
which your measurements indicate. Note
that f from step 1 is not the
same as θ
in the Bragg equation. θ is defined as the angle
between the beam and the crystal planes.
To see the relationship between θ and f,
notice that the angle labeled “a” forms vertical angles with θ.
Both rings are due to first order Bragg
diffraction. The different diameters are
due to different distances between the planes doing the diffracting. The planes responsible for each ring are as
shown. (Graphite has a hexagonal atomic
arrangement.) Calculate the wavelength
which your measurements indicate. Note
that f from step 1 is not the
same as θ
in the Bragg equation. θ is defined as the angle
between the beam and the crystal planes.
To see the relationship between θ and f,
notice that the angle labeled “a” forms vertical angles with θ.
Average the two wavelengths
(one from each ring).
For comparison to these
wavelengths based on measurement, calculate the wavelengths based on de
Broglie's theory. In the homework, you
will show that this is λ = (12.26 Ǻ) / ![]() , where V is the accelerating voltage. (This comes from making some substitutions
into λ
= h / p.)
, where V is the accelerating voltage. (This comes from making some substitutions
into λ
= h / p.)
In your conclusion, compare
the de Broglie wavelength to what was actually measured. (For the measured λ's uncertainty, average the percent uncertainties in the
two diameters used to find it. The
uncertainty in the λ from de Broglie's formula is small.)
PHY 133 Experiment
6B: Electron Diffraction
|
V |
D inner |
D outer |
fi |
fo |
|
|
+ |
+ |
|
|
|
|
+ |
+ |
|
|
|
|
+ |
+ |
|
|
|
λi |
λo |
λave |
de
Broglie λ |
|
|
|
+ |
|
|
|
|
+ |
|
|
|
|
+ |
|
Sample
calculation of f:
Sample
calculation of λ:
Sample calculation of λ’s
uncertainty: